Abstract
Introduction
Internal tandem duplication (ITD) and tyrosine kinase domain (TKD) mutations (m) in FLT3 occur in about 30% of the patients (pts) with newly diagnosed AML. FLT3m are associated with a higher risk of relapse and inferior overall survival (OS). Outcomes remain poor in older/unfit pts with FLT3m AML; with expected median OS of 8-12 months with combinations of low intensity chemotherapy (LIC) with FLT3 inhibitors (FLT3i) or with venetoclax (VEN) [Ohanian et al. AJH, 2018; Konopleva et. al. ASH, 2020). In this current study, our goal was to analyze outcomes in newly diagnosed older/unfit pts with FLT3m AML treated with LIC + FLT3i (doublet regimen) vs. LIC + VEN + FLT3i (triplet regimen) on clinical trials at our institution.
Methods
We identified 87 older or unfit adult pts with newly diagnosed FLT3-m (ITD and/or TKD) AML treated on FLT3i-based LIC clinical trials between 6/2012-3/2021 (Figure 1). All pts had at least two or more bone marrow (BM) assessments including at baseline, end of the first cycle of therapy, and/or later during therapy. MRD assessments were performed by in-house multicolor flow cytometry (MFC) (sensitivity of 10 -4) and multiplex polymerase chain reaction (PCR) (sensitivity of 10 -2-10 -3) for ITD and D835.
Results
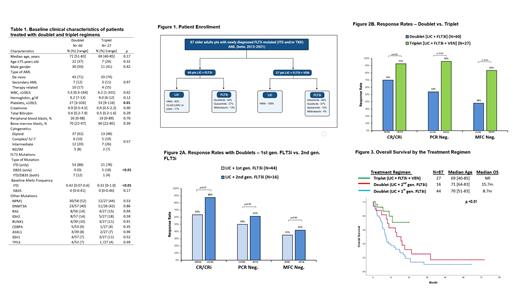
Of the 87 pts with newly diagnosed FLT3m AML, 60 (69%) and 27 (31%) received doublet and triplet regimens, respectively. Baseline clinical characteristics, including age, WBC, organ function, cytogenetics, ECOG PS and molecular aberrations, were generally similar between patients treated with doublet vs triplet (Table 1).
Of the 60 pts who received LIC (HMA 83%, LDAC-based 17%) + FLT3i doublets, 44 (73%) received a first-generation FLT3i (36 sorafenib, 8 midostaurin) and 16 (27%) second-generation FLT3i (quizartinib). Our analysis showed no statistically significant difference in CR/CRi and FLT3-PCR or MFC negativity rates in patients treated with first or second-generation FLT3i based LIC doublets (Figure 2A). There was no statistically significant OS difference between patients treated with first- vs. second-generation FLT3i doublet regimens (P=0.19).
In the triplet group, 12 (44%), 10 (37%), 4 (15%) and 1 (4%) pts received gilteritinib, sorafenib, quizartinib and midostaurin combined with HMA-VEN, respectively. Triplet HMA-VEN-FLT3i was associated with significantly higher CR/CRi (93% vs 70%, P=0.02), FLT3-PCR (96% vs 54%, P<0.01), and MFC negativity (83% vs 38%, P<0.01) rates than doublet regimens (Figure 2B). The 60-day mortality was similar between triplet vs doublet; 7% (n=2) vs 10% (n=6), respectively.
The median follow-up time was shorter in the triplet arm than in the doublet arm: 12 vs. 63 months (p<0.01). The median OS was better with the HMA-VEN-FLT3i triplets compared with the HMA-FLT3i doublets (not reached (NR) vs 9.5 months, P<0.01). The median OS in patients treated with triplets vs second-generation FLT3i doublets vs first-generation FLT3i doublets was NR vs 15.7 vs 8.7 months (P<0.01) (Figure 3).
8 (29%) and 6 (10%) pts went to SCT after triplet vs doublet, respectively. A landmark analysis at 4-month (n=50) demonstrated that pts who received ASCT in CR1 had superior OS than patients who did not receive ASCT in CR1 ( NR vs 19 months, P=0.01).
Conclusions
First- and second-generation FLT3i-based doublet regimens were associated with comparable response rates and survival of 9-16 months in older adults with newly diagnosed FLT3 mutated AML. The HMA-VEN-FLT3i combination significantly improved CR/CRi rates, FLT3-PCR and MFC MRD rates as well as OS, without increasing early mortality in this retrospective analysis. These findings suggest the need for prospective validation of HMA-VEN-FLT3i triplets in older/unfit AML.
Yilmaz: Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Kantarjian: AbbVie: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Jazz: Research Funding; Amgen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Aptitude Health: Honoraria; Astellas Health: Honoraria; BMS: Research Funding; Ascentage: Research Funding; Precision Biosciences: Honoraria; NOVA Research: Honoraria; KAHR Medical Ltd: Honoraria; Ipsen Pharmaceuticals: Honoraria; Astra Zeneca: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Short: AstraZeneca: Consultancy; Astellas: Research Funding; NGMBio: Consultancy; Takeda Oncology: Consultancy, Research Funding; Novartis: Honoraria; Jazz Pharmaceuticals: Consultancy; Amgen: Consultancy, Honoraria. Konopleva: Ascentage: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Ablynx: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; KisoJi: Research Funding; Stemline Therapeutics: Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Cellectis: Other: grant support; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; AstraZeneca: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support. Kadia: AstraZeneca: Other; Astellas: Other; Genfleet: Other; Ascentage: Other; Cellonkos: Other; Sanofi-Aventis: Consultancy; Pulmotech: Other; Pfizer: Consultancy, Other; Novartis: Consultancy; Liberum: Consultancy; Jazz: Consultancy; Genentech: Consultancy, Other: Grant/research support; Dalichi Sankyo: Consultancy; Cure: Speakers Bureau; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; Aglos: Consultancy; AbbVie: Consultancy, Other: Grant/research support. DiNardo: Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; Takeda: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria; AbbVie: Consultancy, Research Funding; Foghorn: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Forma: Honoraria, Research Funding. Borthakur: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; ArgenX: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Ryvu: Research Funding; Protagonist: Consultancy; University of Texas MD Anderson Cancer Center: Current Employment; Astex: Research Funding; GSK: Consultancy. Pemmaraju: LFB Biotechnologies: Consultancy; Incyte: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Clearview Healthcare Partners: Consultancy; Affymetrix: Consultancy, Research Funding; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Samus: Other, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Sager Strong Foundation: Other; Plexxicon: Other, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Cellectis S.A. ADR: Other, Research Funding; CareDx, Inc.: Consultancy; Aptitude Health: Consultancy; Springer Science + Business Media: Other; Roche Diagnostics: Consultancy; DAVA Oncology: Consultancy; MustangBio: Consultancy, Other; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Issa: Syndax Pharmaceuticals: Research Funding; Novartis: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding. Jain: Bristol Myers Squibb: Honoraria, Research Funding; TG Therapeutics: Honoraria; Precision Biosciences: Honoraria, Research Funding; Beigene: Honoraria; Incyte: Research Funding; Aprea Therapeutics: Research Funding; Fate Therapeutics: Research Funding; Janssen: Honoraria; Pfizer: Research Funding; AstraZeneca: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Servier: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Pharmacyclics: Research Funding. Takahashi: Symbio Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Celgene/BMS: Consultancy; GSK: Consultancy. Sasaki: Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees. Loghavi: Abbvie: Current equity holder in publicly-traded company; Curio Sciences: Honoraria; Gerson Lehrman Group: Consultancy; Guidepoint: Consultancy; Peerview: Honoraria; Qualworld: Consultancy. Andreeff: Medicxi: Consultancy; Senti-Bio: Consultancy; Amgen: Research Funding; Syndax: Consultancy; Oxford Biomedica UK: Research Funding; ONO Pharmaceuticals: Research Funding; Karyopharm: Research Funding; Aptose: Consultancy; Daiichi-Sankyo: Consultancy, Research Funding; AstraZeneca: Research Funding; Novartis, Cancer UK; Leukemia & Lymphoma Society (LLS), German Research Council; NCI-RDCRN (Rare Disease Clin Network), CLL Foundation; Novartis: Membership on an entity's Board of Directors or advisory committees; Breast Cancer Research Foundation: Research Funding; Glycomimetics: Consultancy; Reata, Aptose, Eutropics, SentiBio; Chimerix, Oncolyze: Current holder of individual stocks in a privately-held company. Ravandi: AstraZeneca: Honoraria; AbbVie: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prelude: Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Novartis: Honoraria; Xencor: Honoraria, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. Daver: Abbvie: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Novimmune: Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Novartis: Consultancy; Genentech: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Glycomimetics: Research Funding; Amgen: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Astellas: Consultancy, Research Funding; Hanmi: Research Funding; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal